The phosphate rock main market is the production of phosphate fertilizer products such as ammonium phosphates and superphosphates, which accounts for some 90% of the world phosphate rock consumption. Phosphorous plays an important role in root development and in the synthesis of protein, fats and carbohydrates. The rest is consumed as animal feed and in a variety of industrial/technical applications (for phosphoric acid required for detergents and cleaners, food production, metal cleaning, etc.).
Sedimentary deposits provide between 80-90% of the world phosphate rock production, containing francolite–a carbonate-fluorapatite. Francolites with high carbonate for P substitution are the most highly reactive and the most suitable for direct application as fertilizers or soil amendments, but this usage is quite limited and it is estimated that world consumption is less than two million tonnes per annum. Some 10-20% of world phosphate production is mined from igneous deposits. Relative to sedimentary pebble rocks, igneous phosphates have a macrocrystalline structure and are denser and less porous. More than 30 countries produce phosphate rock for commercial purposes, with the top 12 countries supplying over 90% of all the material.
Lower grade rock undergoes a beneficiation process to remove impurities creating an improved quality product with higher phosphate content. The methods employed to beneficiate phosphate rock consist of washing, grinding, flotation (to isolate phosphate bearing ore from certain impurities) and drying. This beneficiation process usually yields a concentration of around 1.5 times, but higher ratios are possible with some rocks. The targeted result of the beneficiation process is a phosphate concentrate ranging from 28% to 35% P2O5.
There is a limited usage of phosphate rock applied directly as a fertilizer. The use of rock phosphate for direct application as fertilizer depends on its level of solubility in the acidic soil. This application is dependent up on the structure and chemical composition of the rock. Mineralogical tests should be done to assess the suitability for direct application. It is stated that carbonate radical is responsible for the reactivity of directly applied P2O5 in the rock.
Sometimes the rock is partially acidulated – called PAPR – a process that converts the insoluble tricalcium phosphate of phosphate rock into a mixture of water-soluble monocalcium phosphate and citrate-soluble dicalcium phosphate. The extent of acidulation depends on factors like the type of acid, acid-rock ratio, temperature, time of reaction, and the proportion of apatite and non-apatite materials in the rock. Different acids have been used and they include phosphoric acid, sulphuric, hydrochloric, nitric, carbonic, oxalic, citric and acetic acids.
The physical characteristics of a rock can sometimes limit its acceptability both for economic and environmental reasons. The hardness of a rock together with the particle size distribution and the type of grinding equipment used will determine the energy used in getting the rock fine enough for the processing stage. Hard rocks and those with angular silica grains increase substantially the wear on grinding equipment. The size distribution of the particles also affects the rock’s handling characteristics. Rocks with too many “fines” will be dusty causing P205 losses and environmental pollution. The water content of concentrates is generally kept above 1.5% to limit dusting and below 2.5% for economic shipping. The pore spacing of the rock also determines its handling characteristics, for instance igneous rock types have few pore spaces and may be saturated with moisture at only 1-2% H20.
The quantity of certain chemicals contained within a rock directly affects its usefulness for the manufacture of phosphoric acid and downstream fertilizers. As well as affecting the process directly, many of these constituents interreact to produce other effects, some beneficial, but mostly detrimental to the reaction. A key factor is the calcium sulphate content of the rock, the remaining CaO level will be directly related to the consumption of sulphuric acid. Seawater washing during rock beneficiation and insufficient freshwater rinsing can be a cause of high and fluctuating Cl levels. As an indication of carbonate levels in the rock, CO2 high values can indicate a tendency to foam during the reaction. Heavy metal content of phosphate products seriously impact the composition of animal feed and food grade products (for example the high cadmium content of the Togolese rock ouput, gravely affects its export possibilities).
Phosphate Rock Production
2012 = About 200 million tonnes rock
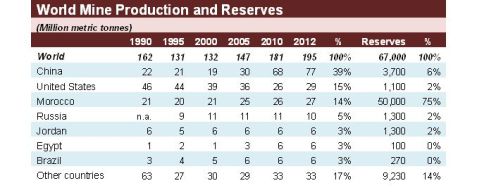
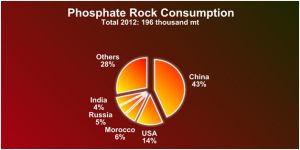
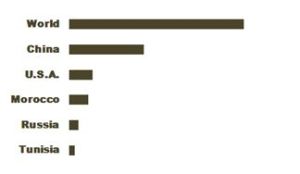
China is the biggest world producer of phosphate rock.
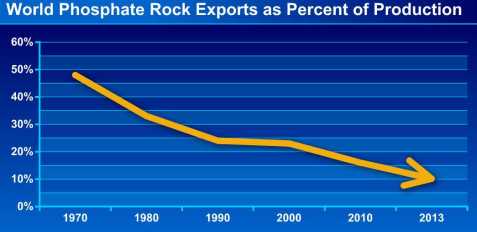
There is a historic trend by which the phosphate fertilizer manufacturing goes from areas lacking the raw material, to those who mine it. So it happens that if in the beginning of the seventies trade of phosphate rock neared half of its production, today it is around a tenth.
In world phosphate rock trade, the Moroccan governmental producer OCP (Office Cherifien des Phosphates) is the key player with more than a third of the world exports.
In 2011, the five largest rock importers were India, USA, Indonesia, Belgium and Brazil; in 2012 they were India, USA, Indonesia, Brazil and Poland.
Morocco has about a three quarters of the world phosphate rock reserves, they are the second world producer and the biggest exporter, accounting for more than a third of the world exports.
India is the largest world importer of phosphate rock, and Jordan is its main supplier.
A key indicator for the rock price levels are the DAP price developments:
The above chart uses prices of phosphate rock (Moroccan), 70% BPL, contract, f.a.s. Casablanca, and DAP, bulk, f.o.b. US Gulf.